飞凡检测致力于打造国内专业的第三方检测服务公司,不断优化整合市场检测资源,飞凡检测与多家科研院所,如上海同济大学,苏州大学,以及国内众多知名的检测公司进行深度合作,共同为国内制造业服务,为中国的制造业开拓海外市场保驾护航,为我们日常生活的健康安全树立标杆;公司目前工程师百余人,95%以上是本科以上学历;我们合作的实验室通过CNAS,CMA(EZ),所出具的任何一张报告都有CNAS,CMA资质,确保出具的每份报告都具有权威的法律效力。
Introduction to CAR-T Therapy

CAR-T cells are transformed in vitro using retroviral or lentiviral vectors to insert CAR sequences into the genome of the patient's T cells. However, due to the stochastic and unpredictable nature of viral vector-mediated insertion, it may affect the normal expression of genes around the insertion site, or even stimulate proto-oncogenes, triggering uncontrolled cell proliferation and cloning.Linker-Mediated (LM-PCR) method is based on a high-throughput sequencing platform combined with the lentiviral LTR-specific primer nested PCR, which can analyze the DNA flanking the integration site by The LM-PCR method is based on a high-throughput sequencing platform combined with the lentiviral LTR-specific primer nested PCR technology, which can be used to search for viral insertion sites at the genome-wide level by analyzing DNA sequences flanking the integration site.
For the risk assessment of viral vector insertion sites, the U.S. FDA has put forward the requirements for this test in several guidelines. China's CDE has also put forward clear requirements for risk assessment related to genome integration in the Technical Guidelines for Non-clinical Research and Evaluation of Gene Therapy Products (Trial), Technical Guidelines for Non-clinical Research of Gene Modified Cell Therapy Products (Trial), and Technical Guidelines for Long-Term Follow-up Clinical Research of Gene Therapy Products, which will be promulgated in 2021. For more information, please contact: 13524733472
Our Technical Services


Our Advantages

Precise enrichment at the whole genome level
Simultaneous detection of 3'LTR+5'LTR to complement each other
Comprehensive methodology validation for monoclonal, oligoclonal and clinical samples
Diversity of analysis content to meet the reporting requirements
For more information, please contact us at 13524733472
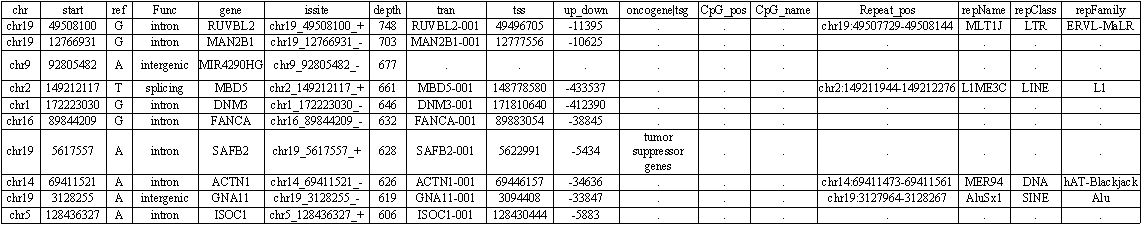
Some of our cases





References
1.Technical Guidelines for Non-clinical Research and Evaluation of Gene Therapy Products (for Trial Implementation).2021 Edition
2.Technical Guidelines for Non-clinical Research on Gene Modified Cell Therapy Products (for Trial Implementation).2021 Edition
3. Technical Guidelines for Long-term Follow-up Clinical Studies of Gene Therapy Products. 2021 Edition
4. Technical Guidelines on Clinical Risk Management Program for Chimeric Antigen Receptor T Cell (CAR-T) Therapeutic Products Declared for Marketing. 2021 Edition
5. Chinese Pharmacopoeia, 2020 Edition.

飞凡检测致力于打造国内专业的第三方检测服务公司,不断优化整合市场检测资源,飞凡检测与多家科研院所,如上海同济大学,苏州大学,以及国内众多知名的检测公司进行深度合作,共同为国内制造业服务,为中国的制造业开拓海外市场保驾护航,为我们日常生活的健康安全树立标杆;公司目前工程师百余人,95%以上是本科以上学历;我们合作的实验室通过CNAS,CMA(EZ),所出具的任何一张报告都有CNAS,CMA资质,确保出具的每份报告都具有权威的法律效力。